The main ideas for this past week were figuring out how to find the correct calculations for our previous known information, through PTVn lab tables. Branching off of the our previous weeks’ main focus (PTVn Labs), this past week we focused on studying the relationship between pressure, temperature and pressure.
In order to find the proper calculations (pressure, temperature, volume, numbers of particles), our class worked through two PTVn problem packets. When working through each problem with our table members, we were instructed to conduct whiteboard discussions for each word problem to ensure everyone comprehended the information. To thoroughly explain how I came to understand this week’s lesson, here is a picture:  Here I am going to work through one of the problems that our class did in order to fully explain the lesson. In the table we have initial data, final data and the effect (positive or negative) slots to record our information. The word problem gives us the proper numbers that allow us to just plug the information into the table.
Here I am going to work through one of the problems that our class did in order to fully explain the lesson. In the table we have initial data, final data and the effect (positive or negative) slots to record our information. The word problem gives us the proper numbers that allow us to just plug the information into the table.  Once plugging in the provided information, I know that I do not have to calculate the temperature or number or particles for this problem so I simple cross those slots out. Keeping in mind that our goal for this problem is to find the final volume, we can solve this problem by making an equation. To make this equation, I thought of these numbers as ratios. It was easier for me to think of them as ratios, that way I could properly set up the fractions in the equation. For every 15.5mL, there is 14.7psi and we do not know the final volume but we know there is 10.9psi. To set this up it would be:15.5mL / x = 14.7psi / 10.9psi. Now, from our previous weeks lesson we know that as pressure decreases volume increases ( and vice versa). This means the largest number of pressure needs to go on top of the fraction because the largest number would cause the increase in volume:
Once plugging in the provided information, I know that I do not have to calculate the temperature or number or particles for this problem so I simple cross those slots out. Keeping in mind that our goal for this problem is to find the final volume, we can solve this problem by making an equation. To make this equation, I thought of these numbers as ratios. It was easier for me to think of them as ratios, that way I could properly set up the fractions in the equation. For every 15.5mL, there is 14.7psi and we do not know the final volume but we know there is 10.9psi. To set this up it would be:15.5mL / x = 14.7psi / 10.9psi. Now, from our previous weeks lesson we know that as pressure decreases volume increases ( and vice versa). This means the largest number of pressure needs to go on top of the fraction because the largest number would cause the increase in volume: 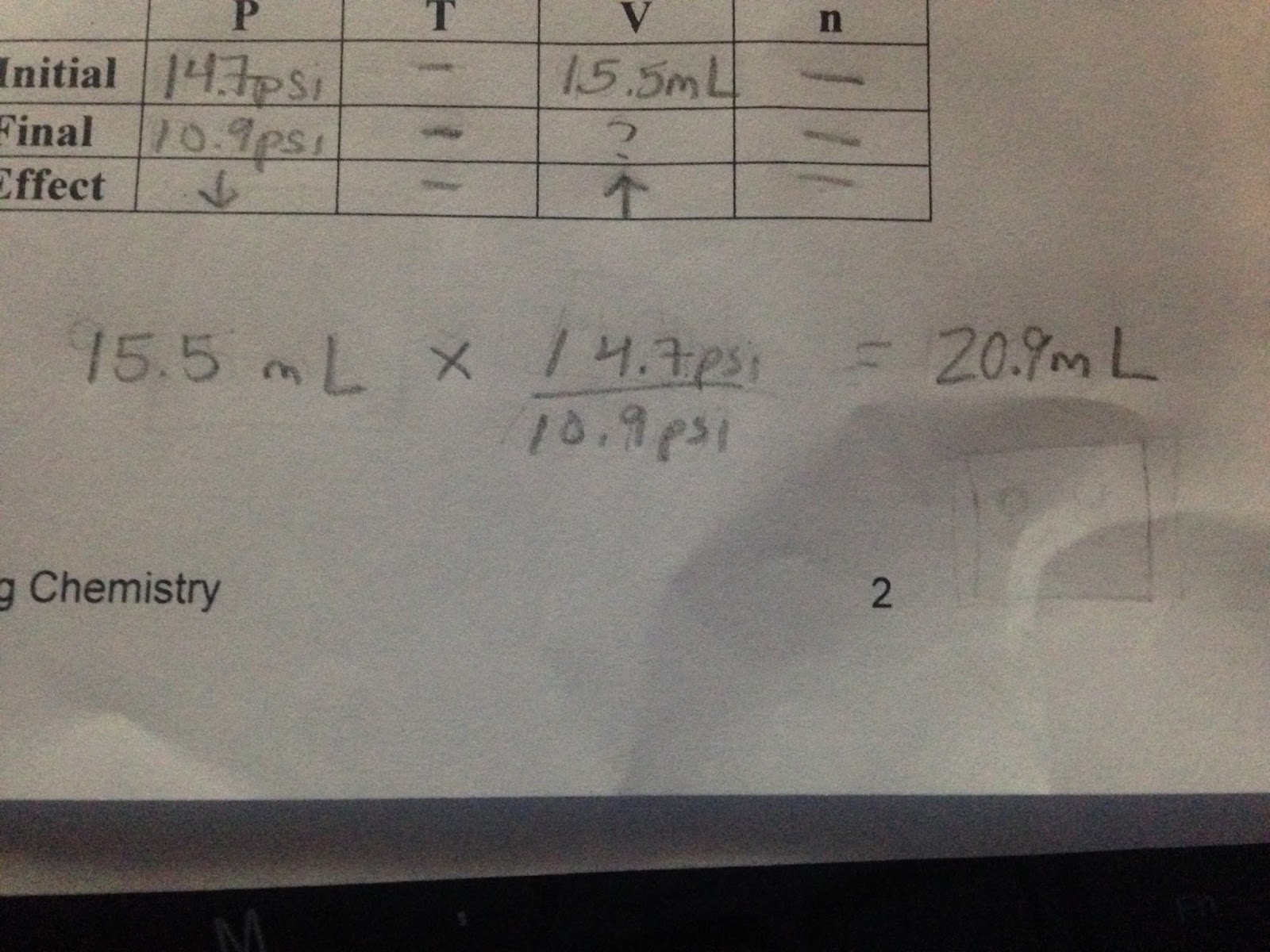 Once solving this equation my calculator (95.5 x 14.7 / 10.9), the final volume results were 20. 9mL. From a lesson learned in previous weeks, I also know that our final answer must match in significant figures with the initial information. In this case, the significant figures match. We know this because the initial volume has 3 significant figures and due to our final volume having a zero that is “sandwiched”, also makes the number have 3 significant figures. After figuring out this information, we can thoroughly understand what is taking place in the word problem by drawing out a model:
Once solving this equation my calculator (95.5 x 14.7 / 10.9), the final volume results were 20. 9mL. From a lesson learned in previous weeks, I also know that our final answer must match in significant figures with the initial information. In this case, the significant figures match. We know this because the initial volume has 3 significant figures and due to our final volume having a zero that is “sandwiched”, also makes the number have 3 significant figures. After figuring out this information, we can thoroughly understand what is taking place in the word problem by drawing out a model: 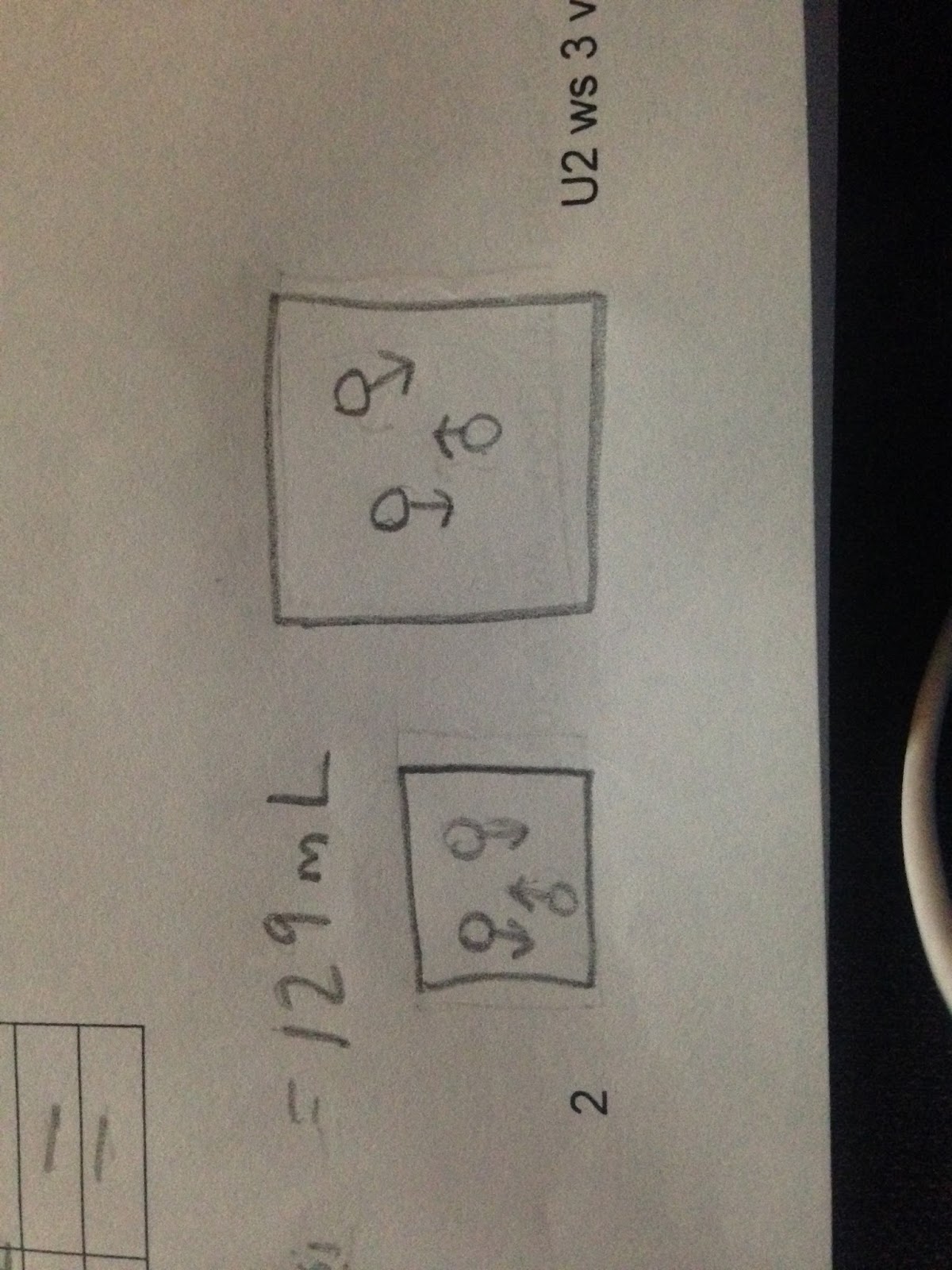 We know that the effect of the volume increased by not only the dependent of pressure, but the resulting answer of our equation. Knowing this information, we can conclude the final volume to be much larger than initial volume. Another piece of information that is important to know when drawing the model is the temperature and number of particles. We know that temperature and the number of particles were not affected. This means they were a constant variable and did not need to be altered in the model. To show this effect in the final and initial models, I drew the same amount of particles and same size arrows. We continued this lesson with various different scenarios and problems.
We know that the effect of the volume increased by not only the dependent of pressure, but the resulting answer of our equation. Knowing this information, we can conclude the final volume to be much larger than initial volume. Another piece of information that is important to know when drawing the model is the temperature and number of particles. We know that temperature and the number of particles were not affected. This means they were a constant variable and did not need to be altered in the model. To show this effect in the final and initial models, I drew the same amount of particles and same size arrows. We continued this lesson with various different scenarios and problems.
The second topic we focused on was studying for the unit 2 test. To prepare for this test we worked through the review guide and discussed the questions with our table groups.
My thoughts of this weeks were positive. I didn’t realize how much our previous lessons would be helpful for us until coming into this week's lesson. I also enjoyed how much we collaborated with our groups more. I liked being able to work through problems and write everything out on our whiteboards. Lastly, I found it helpful to work through some of the review guide in class with our classmates because we were able to thoroughly discuss each problem with one another. My overall comprehension of this week’s lesson from 1-10 would be a 9. I feel confident about having to solve the PTVn tables and would not mind doing them individually. My thoughts of the review guide are a little on the less confident side, but I think if I went through my journal I would be more confident for the test. Overall, I think my progress in the class is improving and I look forward for our next upcoming lessons.
No comments:
Post a Comment